
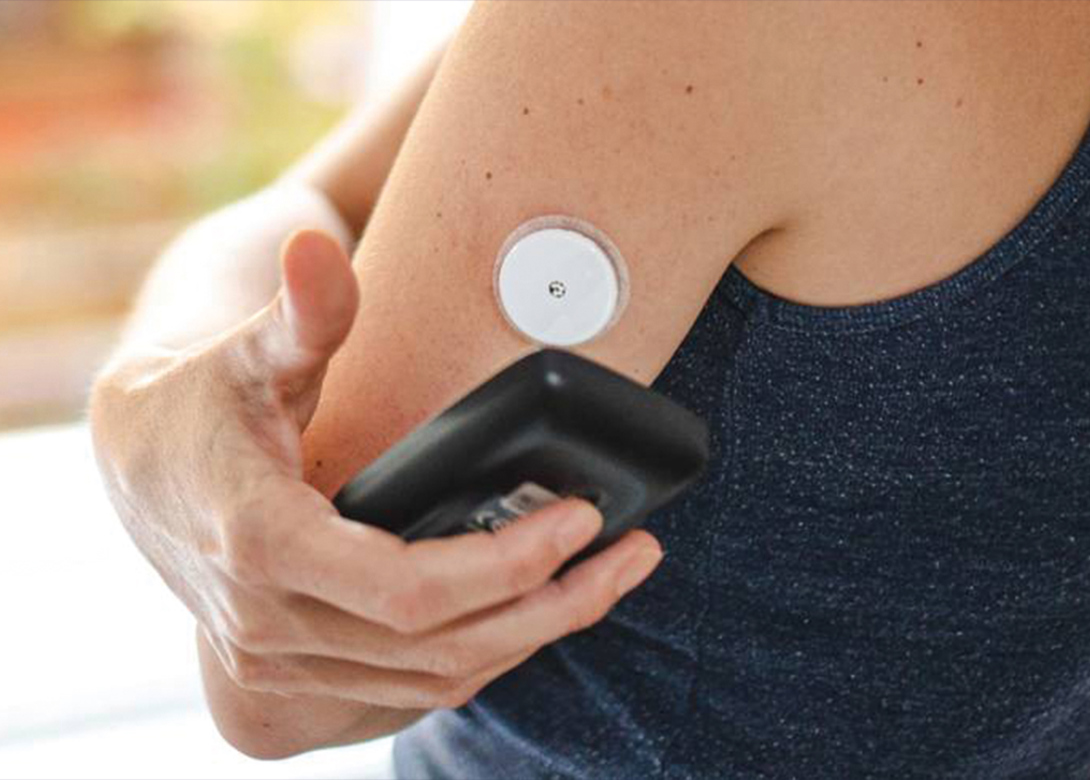
Dymax, leading global manufacturer of rapid curing materials and equipment, has introduced the 2000-MW series of light curable adhesives specifically developed to address customer requirements and market trends within the rapidly evolving wearable medical devices market.
This new range of structural medical adhesives pass ISO 10993-10 for sensitisation and irritation. Free from TPO, a material of concern, and made without IBOA, a known skin irritant, these adhesives address device manufacturers’ concerns about skin proximity and sensitivity.
Designed for bonding SS, PC, PI, PVC, TPU, and low surface energy substrates, the adhesives are also engineered for reliability with excellent adhesion and aging performance. The 2000-MW series cures with broad spectrum light energy and some products provide durable protection during autoclave sterilisation.
“Staying true to our core values while addressing the evolving needs of our customers, Dymax continues to develop innovative light curable adhesives. Removing materials of concern with the introduction of the 2000-MW series of products is our first step toward the elimination of problematic ingredients,” said Mike Ford, global business development director, medical for Dymax.
These adhesives are environmentally friendly, one part formulations, solvent free, and meet ISO 10993-5 cytotoxicity biocompatibility requirements. Typical applications include devices worn in close proximity to the skin such as continuous glucose monitors, large volume injectors, patient monitoring devices, and pain management devices. Fluorescing grades are available for high-speed automated vision systems used for quality control purposes.

Having spent a decade in the fastener industry experiencing every facet – from steel mills, fastener manufacturers, wholesalers, distributors, as well as machinery builders and plating + coating companies, Claire has developed an in-depth knowledge of all things fasteners.
Alongside visiting numerous companies, exhibitions and conferences around the world, Claire has also interviewed high profile figures – focusing on key topics impacting the sector and making sure readers stay up to date with the latest developments within the industry.





